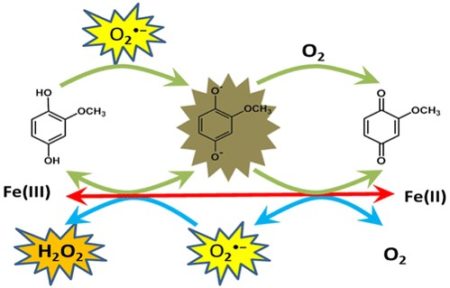
Schematic shows how Iron (Fe) can start a catalytic pathway that accelerates the oxidation of natural organic matter by oxygen (O2). The important reactive oxygen species intermediates are also show, hydrogen peroxide (H2O2) and superoxide (O2-●)
Scientific Achievement
We were able to experimentally demonstrate and numerically model a new pathway by which natural organic matter can be oxidized (decomposed) by oxygen. We showed that a type of organic matter that was not directly oxidized by oxygen rapidly oxidized with the addition of catalytic amounts of iron.
Significance and Impact
The pathways and rates of oxidation of natural organic matter is an important part of the global carbon cycle. If modeling of global carbon cycling is to be accurate it is important that we have a clear understanding of all the significant pathways. This work contributes to that need.
Research Details
- We used a model organic compound to represent natural organic matter.
- We measured the rate of oxidation of this compound in the presence and absence of iron.
- We used a numerical kinetic model to show that iron initiates an important catalytic oxidation pathway that relies on important reactive oxygen species intermediates, hydrogen peroxide (H2O2) and superoxide (O2-●)
Citation
Yuan, X.; Davis, J. A.; Nico, P. S. (2016), Iron-Mediated Oxidation of Methoxyhydroquinone under Dark Conditions: Kinetic and Mechanistic Insights, Environmental Science & Technology, 50(4), 1731-1740 DOI: 10.1021/acs.est.5b03939.