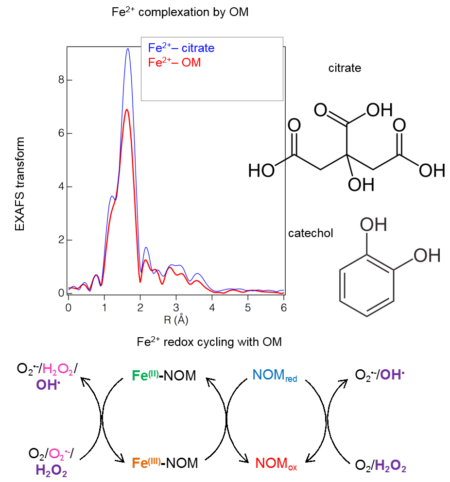
Top: EXAFS data revealed that iron(II) is dominantly complexed by carbonate groups in organic matter (OM). Bottom: Iron(II) bound to reduced OM undergoes redox cycling upon exposure to O2 that generates reactive oxygen species.
Scientific Achievement
Daugherty et al. identified the organic functional groups in soil organic matter (OM) that preferentially bind to reduced form of dissolved iron and showed that reduced OM can stabilize Fe(II) by functioning both as redox buffer, which may help explain the widespread presence of Fe(II) in oxic circumneutral waters.
Significance and Impact
Iron (Fe) bioavailability depends upon its solubility and oxidation state, which are strongly influenced by association NOM. The knowledge of iron(II) binding mechanisms by OM enhances our models for iron(II) transport, redox cycling and bioavailability in terrestrial ecosystems.
Research Details
- Use of extended X-ray absorption fine structure (EXAFS) spectroscopy to determine the coordination environment of Fe(II) associated with NOM
- Linear combination analysis of EXAFS data determined that Fe(II) was complexed primarily by carboxyl functional groups in reduced NOM. Catecholate groups play a secondary role.
- Reduced OM stabilizes iron(II) against oxidation by O2 for at least 12 hours
Citation
Daugherty, E.L., B. Gilbert, P.S. Nico and T. Borch (2017) Complexation and Redox Buffering of Iron(II) by Dissolved Organic Matter. Environmental Science and Technology 51, 11096-11104. DOI: 10.1021/acs.est.7b03152