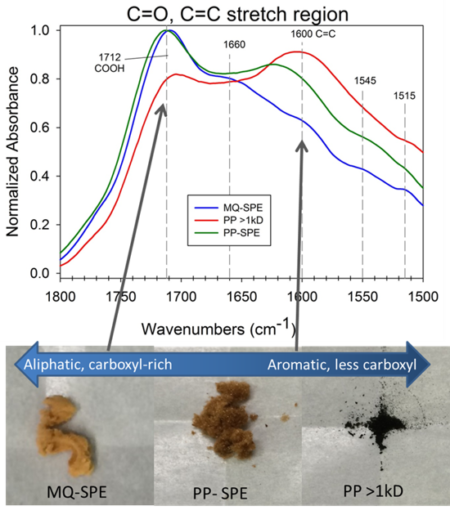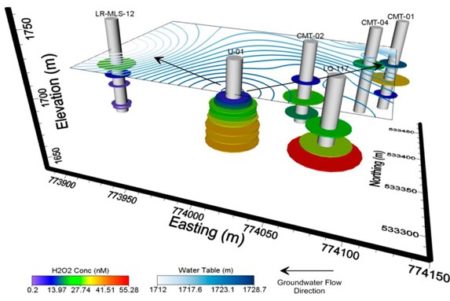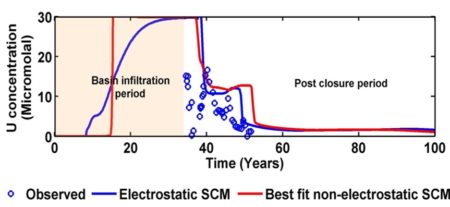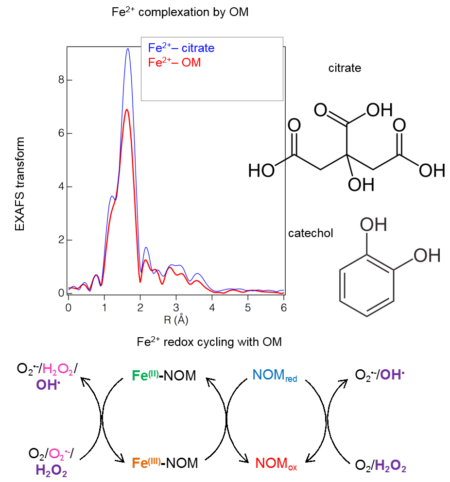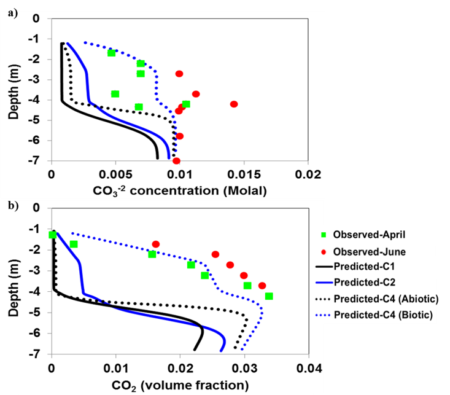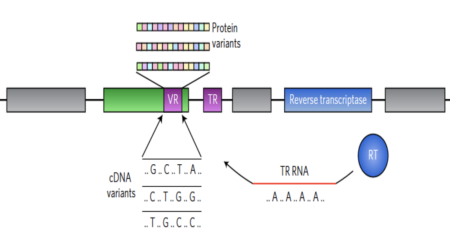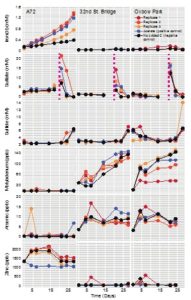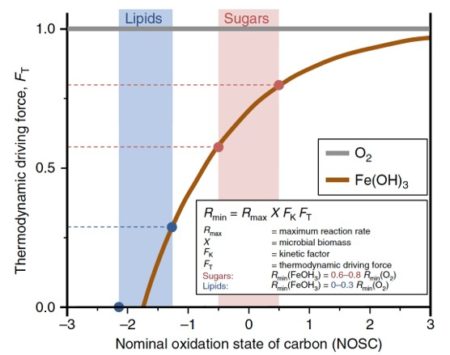
Figure shows how certain kinds of organic molecules, e.g. Sugars or lipids can only be oxidized by microbes with certain electron donors. In the absence if oxygen, lipids can not be broken down for energy
Scientific Achievement
This work demonstrated that changes in redox conditions over small spatial distances can have a significant impact on the types of organic molecules that are decomposed by soil microbes
Significance and Impact
This is significant because the controls on the decomposition of soil carbon are one of the most important and most poorly understanding regulators of global carbon cycling. By demonstrating the importance of an under appreciated control mechanism. This work has advanced the overall field of carbon cycling.
Research Details
- The experiment unitized a simply reactor with different textured soil material.
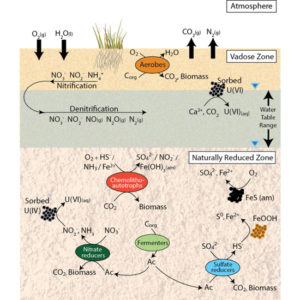
- By changing the soil texture the degree of O2 permeation is impacted which in turn creates anaerobic microsites in the system
- The results showed that these anaerobic site had much slower carbon decomposition than the other locations.
- When making an estimate of how many anaerobic microsite there are in global soils it can be shown that this mode of carbon projecting is globally relevant.
Citation
Keiluweit, M.; Wanzek, T.; Kleber, M.; Nico, P.; Fendorf, S. (2017), Anaerobic microsites have an unaccounted role in soil carbon stabilization, Nature Communications, 8 DOI: 10.1038/s41467-017-01406-6.